

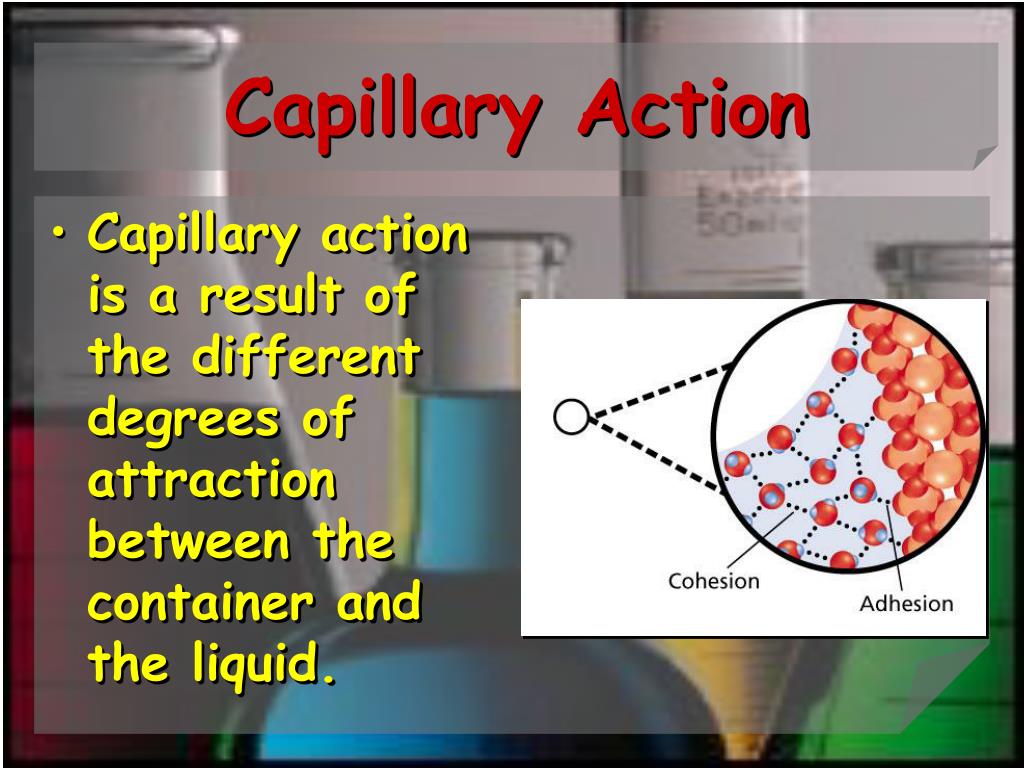
It is sometimes referred to as capillary attraction, capillarity or wicking. Objects that are denser than the liquids can float on top of them without any assistance and cannot sink due to surface tension.Capillary action causes the water in the thinnest tube to rise to a higher level than in the other tubesĬapillary action is a phenomenon associated with surface tension, whereby liquids can travel – horizontally or vertically (against the force of gravity) in small spaces within materials. Most people are aware of the phenomenon of surface tension, but few are aware that it also results from the idea of cohesiveness. The same force holds together raindrops before they descend to the ground. Forces in Capillary Action CohesionĬohesion is the term used to describe the interaction of molecules in a certain medium. A tube’s diameter must be sufficiently tiny for the capillary to form. These forces cause the liquid to be drawn into the tube.

Two kinds of intermolecular forces are adhesion and cohesion. Together, the cohesive forces of the liquid and the adhesive forces between the liquid and the tube material cause capillary action. In terms of capillary rise or fall, the expression for surface tension is, Thus, equating right side of equation 1 and equation 3, we get
The vertical component of the surface tension force must be equal to and opposite to this. This implies that the liquid will experience capillary depression or fall. The angle of contact θ is acute if the capillary tube is maintained vertically in a liquid with a convex meniscus. The higher the liquid rises, the narrower the tube must be (or falls).

The expression for a liquid’s capillary rise or fall is given by the equation above. Consequently, the meniscus’s radius of curvature R is determined by Let r be the radius of the capillary tube and θ be the angle of contact of the liquid. Where, ρ is the density of the liquid and g is acceleration due to gravity. The pressure resulting from the h height liquid column must be equal to the 2T/R concavity-related pressure difference. For capillary rise or fall, use the following formula:Įxpression for capillary rise or fall Method 1: Using Pressure difference Surface tension is another factor that causes a liquid column inside a tiny capillary tube to rise. This occurs as a result of water absorption into the paper’s microscopic spaces between fibers. Similar results occur when a piece of paper is placed on a puddle of water it absorbs the water. Capillarity or capillary action occurs as a result of the intermolecular force of attraction that exists between solids and liquids. Water from the roots and lower levels of the plant is drawn up as the water on the surface of the leaves evaporates.įundamentally, liquids have the ability to be pulled into tiny crevices, as those between sand grain fragments, and to rise into thin tubes. Capillary action also plays a role in the movement of fluids within a plant’s xylem vessels. Groundwater moves through the various soil zones as a result of capillary action. The film that was created on the soil molecules’ outer surface may also start to flow. Difference Between Simple Pendulum and Compound Pendulum.Difference between Gravitational Force and Electrostatic Force.Difference between Static Friction and Dynamic Friction.Difference between Voltage Drop and Potential Difference.Simple Pendulum - Definition, Formulae, Derivation, Examples.Differences between ‘heat capacity’ and ‘specific heat capacity'.Difference between Wavelength and Frequency.Difference between Center of Mass and Center of Gravity.Difference Between Diode And Zener Diode.ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.GATE CS Original Papers and Official Keys.


 0 kommentar(er)
0 kommentar(er)
